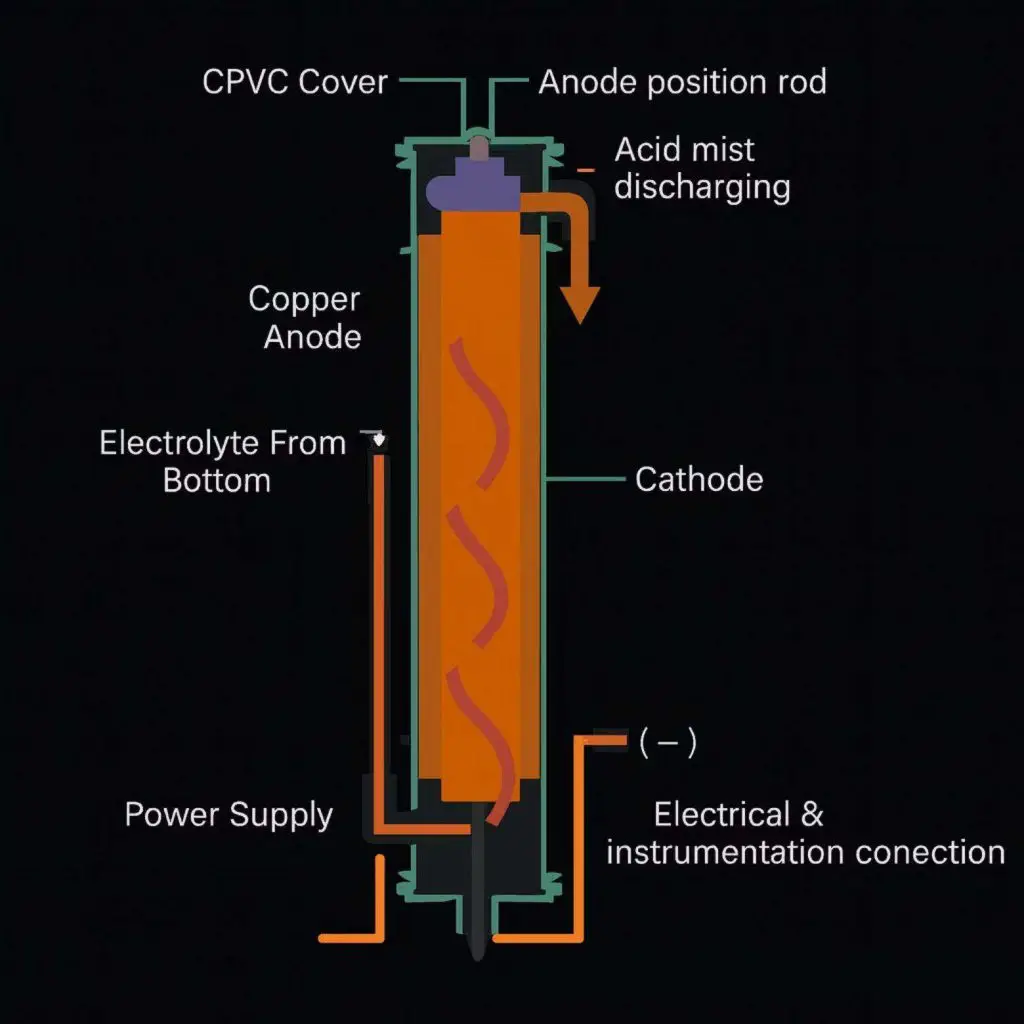
The Purification Process
Electrorefining is an electrochemical process used to purify metals to very high purity levels. It starts with an impure metal anode (often from a smelting process) which is dissolved in an electrolyte solution. An electric current causes the pure metal ions to deposit onto a pure cathode, leaving impurities behind or collecting them as anode slimes.
- Anode: Impure metal that dissolves into the electrolyte.
- Cathode: Thin sheet of pure metal where refined metal deposits.
- Electrolyte: Acidic solution containing metal ions, crucial for conduction.
- Anode Slimes: Valuable residue of less noble metals and precious metals.